- About
-
-

- About
-
ACGIH is a 501(c)(3) charitable scientific organization that advances occupational and environmental health.
-
-
- Subscriptions
-
- Science
-
-

- Science
-
This section has been established to help educate industry, government, and the public on what TLVs and BEIs are, and how TLVs and BEIs may best be used.
-
-
- Career Development
-
-

- Career Development
-
ACGIH is committed to providing its members and other occupational and environmental health professionals with the training and education they need to excel in their profession.
-
-
- Publications
-
-

- Publications
-
ACGIH has publications in many different areas that fit your needs in your field.
-
- Publications Store
- ACGIH Signature Publications
ACGIH Digital Library
If you need to purchase the Digital Library, click here.
If you have purchased and need to access the Digital Library, click here.
-
-
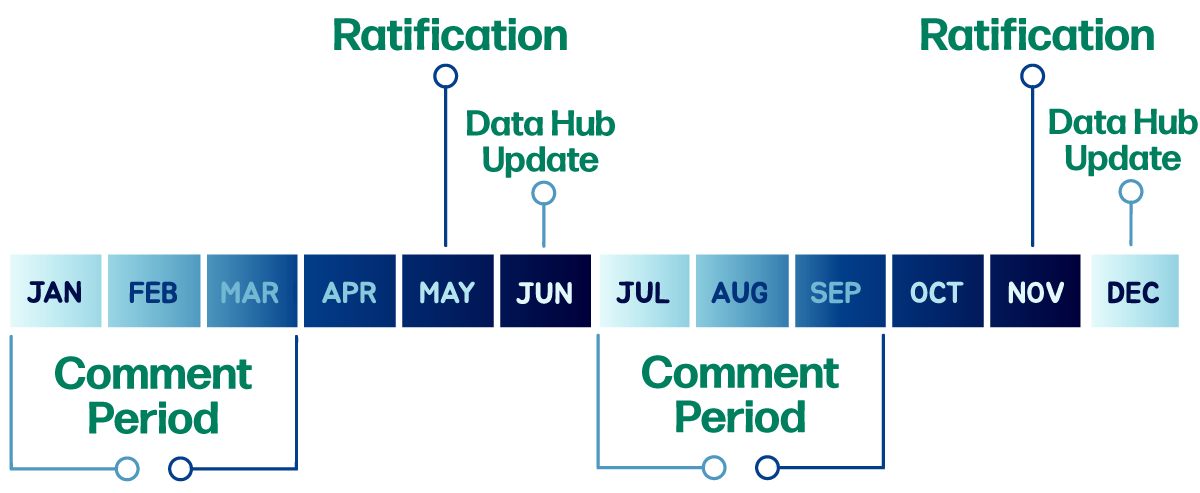
In 2023, ACGIH had updated the TLV development process to better meet the needs of occupational and environmental health professionals. Industry professionals have the opportunity to provide comments twice per year for draft Documentation on the Notice of Intended Changes (NIC) and Notice of Intent to Establish (NIE) lists. The comment periods runs from January 1st to March 31st and July 1st to September 30th, with ratification occurring in May and November, and all changes will be posted online using Data Hub. TLV books will continue to be published at the beginning of every year. This compressed schedule will allow ACGIH scientific committees to finalize critical agents in half the time – providing critical information to the field when it is needed, while still allowing for input on all TLV changes.
Additionally, the under study list is no longer be tiered to allow chemical substances and physical agents to be added throughout the year and worked on immediately. All updates to the under study list will be reflected in ACGIH communications and on the ACGIH website.
Industry professionals have the opportunity to provide comments twice per year for draft Documentation on the Notice of Intended Changes (NIC) and Notice of Intent to Establish (NIE) lists. All substances that are on the NIC currently will have the opportunity to be adopted by the Board of Directors. They are available on our website via Data Hub and in the Publications Store. Click here for current substances and agents list.
All comments can be submitted to the ACGIH Science Group at science@acgih.org following the process outlined on the website.
2025 Documentation – Click here to see substances and agents listing of new Documentation for 2025.
PDF Format is Available for Purchase – PDF versions of all Documentation are available for purchase in the ACGIH Publication Store.*
- Click here for All Documentation.
- Click here for 2025 Documentation.
*Access to the Data Hub Documentation is a complimentary benefit of an ACGIH subscription. PDF formats of the Documentation are NOT complimentary. PDFs are only available for purchase in the ACGIH Publications Store. Subscribers get them for the subscriber discount of $48 per Documentation; $60 for Non-Subscribers.
Click here to Data Hub
Enjoy exclusive benefits including free and discounted publications, conferences and continuing education cour…






