- About
-
-

- About
-
ACGIH is a 501(c)(3) charitable scientific organization that advances occupational and environmental health.
-
-
- Subscriptions
-
- Science
-
-

- Science
-
This section has been established to help educate industry, government, and the public on what TLVs and BEIs are, and how TLVs and BEIs may best be used.
-
-
- Career Development
-
-

- Career Development
-
ACGIH is committed to providing its members and other occupational and environmental health professionals with the training and education they need to excel in their profession.
-
-
- Publications
-
-

- Publications
-
ACGIH has publications in many different areas that fit your needs in your field.
-
- Publications Store
- ACGIH Signature Publications
ACGIH Digital Library
If you need to purchase the Digital Library, click here.
If you have purchased and need to access the Digital Library, click here.
-
-
Download the Comment Submission Form, fill in your comments, and email it to the ACGIH Science Group at science@acgih.org. Please note that this submission form should not be altered other than adding recommendations, rationale, and references, including additional boxes for these items, which should all match the style, font, layout, etc. of this form. Alterations include, but are not limited to changing the font, paragraph, style, and layout of the submission form. Submissions that alter or do not use this submission form may not be accepted.
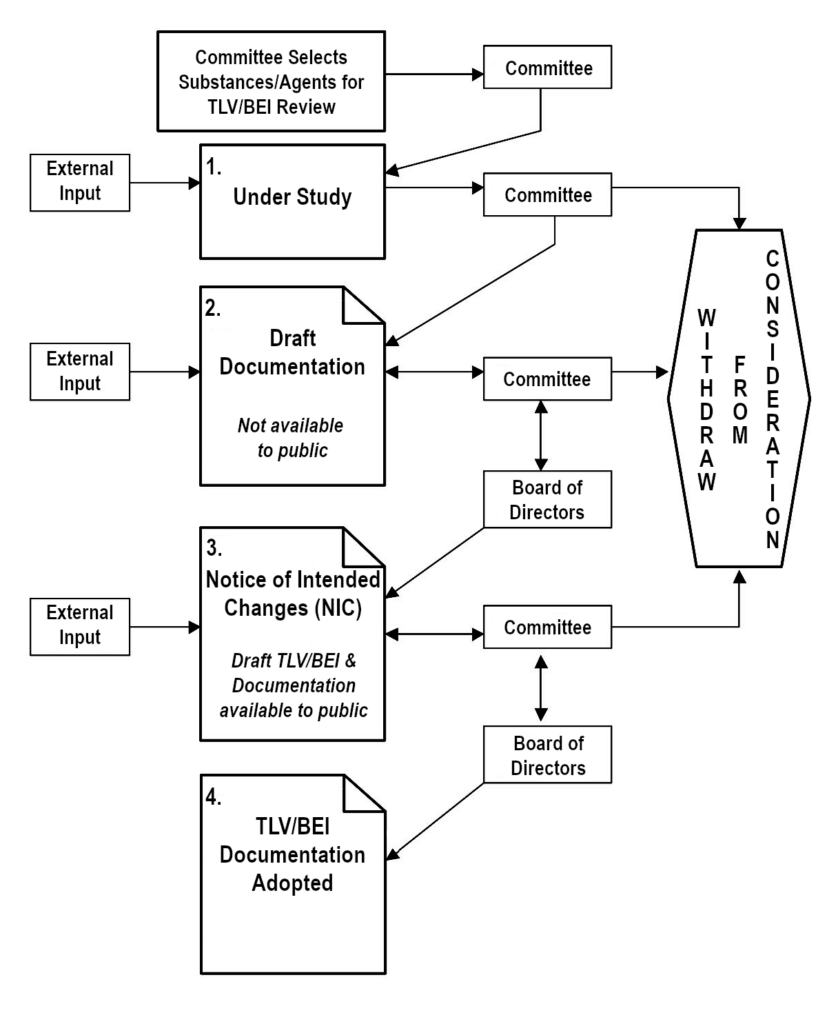
ACGIH encourages interested parties to submit comments and data relevant to the TLV and BEI committee work. This may include substances and agents on Under Study lists, on the NIC/NIE, or for which no TLV or BEI exists. The public may offer input to any TLV or BEI Committee by e-mailing science@acgih.org.
Changes or updates are made to the draft Documentation as necessary. The most effective and helpful comments address specific points within the draft Documentation. If the committee finds or receives substantive data that changes its scientific opinion regarding TLV or BEI values or notations, the committee may revise the proposal(s) and recommend an action to the ACGIH Board of Directors.
Each committee considers only those comments and data that address issues of health and exposure, but not economic or technical feasibility. Comments must be accompanied by peer-reviewed literature that supports the proposed recommendation(s) and rationale(s).
Permission to Use Unpublished Studies
If the data is from unpublished studies, ACGIH requires written authorization from the owner of the studies granting ACGIH permission to (1) use, (2) cite within the Documentation, and (3) release the information, upon request from a third party. Below is a sample permission statement granting ACGIH authorization to use, cite, and release unpublished studies:
[Name], [author or sponsor of the study**] grants permission to ACGIH to use and cite the documents listed below, and to fully disclose them to parties outside of ACGIH upon request. Permission to disclose the documents includes permission to make copies as needed.
Example: Joseph D. Doe, PhD, co-author of the study, grants permission to ACGIH to use and cite the document listed below, and to fully disclose this document to parties outside of ACGIH. Permission to disclose the document includes permission to make copies as needed.
“Effects of quartz status on pharmacokinetics of intratracheally instilled cristobalite in rats [unpublished data]. March 21, 2003.”
**This statement must be signed by an individual authorized to give this permission and should include contact information such as title, company, and email address.
The comment period for an NIC/NIE draft Documentation and its respective TLV(s), notation(s), or BEI(s) will be limited to a firm 3-month period twice a year, running from January 1 to March 31 and July 1 to September 30. All comments received before March 31 will be considered in the spring meeting, and those received before September 30 will be considered in the fall meeting. Draft Documentation will be available for review during the comment period.
Comment Submission Procedures
Electronic submission of all information must be sent to the ACGIH Science Group at science@acgih.org. Download the Word document of the Comment Submission Form. Fill in your comments and email to the ACGIH Science Group. Comments submitted without the Comment Submission Form will not be accepted.
When submitting comments, ACGIH requires that the submission be limited to 10 pages in length, including an executive summary. The submission may include appendices of cited material not included as part of the 10-page limit.
- Chemical Substance or Physical Agent
- Each substance or agent must be submitted in its own document. Submissions that include multiple substances or agents within a single document will not be accepted.
- Comments regarding chemical substances that have a TLV and BEI must have a separate submission for the TLV and another for the BEI. Submissions that include TLV and BEI information in a single document will not be accepted.
- Executive Summary
- This summary has a 250-word limit.
- Recommendations/Actions
- Identify, in a vertical list, specific recommendations/actions being requested.
- Each recommendation/action must be listed as a separate numbered item.
- Rationale
- Provide specific rationale to justify each recommendation/action requested.
- The rationale must directly follow the recommendation/action for which it is intended.
- References
- Provide specific references that directly relate to the specific recommendation/action and rationale. General references, including full websites, will not be accepted.
- References must be peer-reviewed, publicly available literature.
- Unpublished studies may be accepted but must be accompanied by written permission to share the studies with ACGIH and any outside of ACGIH who request to view it.
- If any other items are attached, such as full-text articles, it must include written permission to share with ACGIH for Documentation review.
- Additional Information
- Under Study Chemical Substances or Physical Agents
- Correspondence for items on the under study list will be kept on file until the Documentation is being actively researched or written. Only confirmation of receipt will be sent to commentors.
- New or Adopted Chemical Substances or Physical Agents
- Correspondence for adopted or new Documentation will be sent to the appropriate scientific committee for review. Only confirmation of receipt will be sent to commentors.
- Duplication of Submissions
- Duplicate submissions during the same or following comment periods will not be accepted. However, addendums with additional actions/recommendations, rationale, or references will be accepted.
- Under Study Chemical Substances or Physical Agents
There are several important points to consider throughout the above process.
The appropriate method for an interested party to contribute to the TLV and BEI process is through submitting peer-reviewed and publicly available literature. ACGIH strongly encourages interested parties to publish their studies and not rely on unpublished studies as input to the TLV and BEI process. ACGIH does not commit to deferring consideration of a new or revised TLV or BEI pending the outcome of proposed or ongoing research. Also, the best time to submit comments to ACGIH is in the early stages of the TLV and BEI development process, preferably while the substance or agent is on the Under Study list.
Symposia and Workshops
An additional venue for presenting new data is an ACGIH-sponsored symposium or workshop that provides a platform for public discussion and scientific interpretation. ACGIH encourages input from external parties for suggestions on symposium topics, including suggestions about sponsors and speakers. ACGIH employs several criteria to determine the appropriateness of a symposium. A key criterion is that the symposium must be the most efficient format to present the committee with information that will assist in the scientific judgment used for writing the Documentation and in setting the respective TLVs or BEIs. A symposium topic should be suggested while the substance/agent is under study, as symposia require considerable time, commitment, and resources to develop. Symposium topic suggestions submitted while a substance is on the NIC/NIE will be considered, but this is usually too late in the decision-making process. A symposium topic will not be favorably considered if its purpose is to provide a forum merely for voicing opinions about existing data. Rather, there must be ongoing research, scientific uncertainty about currently available data, or another scientific reason for the symposium. Symposium topic suggestions should be sent to the ACGIH Science Group (science@acgih.org).
ACGIH periodically receives requests from external parties to make a presentation to a committee about specific substances. Such requests are granted by exception. While there are various reasons for this position, the underlying fact is that the committee focuses on data that have been peer-reviewed and published and not on data presented in a private forum. A committee may grant a request when the data are significantly new, have received peer review, are the best vehicle for receipt of the information, and are essential to the committee’s deliberations. The presentation is not a forum to merely voice opinions about existing data. In order for a committee to evaluate such a request, the external party must submit a request in writing that, at a minimum, addresses the following elements: (a) a detailed description of the presentation; (b) a clear demonstration of why the information is important to the committee’s deliberations; and (c) a clear demonstration of why a meeting is the necessary method of delivery. This request must be sent to the ACGIH Science Group (science@acgih.org). Also, the committee may initiate contact with outside experts (a) to meet with the committee to discuss specific issues or to obtain additional knowledge on the subject and (b) to provide written input or review of Documentation. This contact is only done on an as-needed basis, not as a routine practice.
Last revised June 2023
Enjoy exclusive benefits including free and discounted publications, conferences and continuing education courses – all while supporting the TLVs® and BEIs®.






