- About
-
-

- About
-
ACGIH is a 501(c)(3) charitable scientific organization that advances occupational and environmental health.
-
-
- Subscriptions
-
- Science
-
-

- Science
-
This section has been established to help educate industry, government, and the public on what TLVs and BEIs are, and how TLVs and BEIs may best be used.
-
-
- Career Development
-
-

- Career Development
-
ACGIH is committed to providing its members and other occupational and environmental health professionals with the training and education they need to excel in their profession.
-
-
- Publications
-
-

- Publications
-
ACGIH has publications in many different areas that fit your needs in your field.
-
- Publications Store
- ACGIH Signature Publications
ACGIH Digital Library
If you need to purchase the Digital Library, click here.
If you have purchased and need to access the Digital Library, click here.
-
-
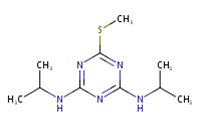
PROMETRYN
PROMETRYN
CAS number: 7287-19-6
Synonyms: A 1114, EINECS 230-711-3, Prometryne, Promethryne, Caparol, G 34161, Gesagard, Primatol Q, Prometrex, Selectin, 2-Methylthio-4,6-bis(isopropylamino)-s-triazine, N, N’-Bis(1-methylethyl)-6-methylthio-1,3,5-triazine-2,4-diamine.
Molecular formula: C10H19N5S
Chemical structure:
To view TLV and Documentation, you must be logged in as an ACGIH member or have previously purchased the documentation.





